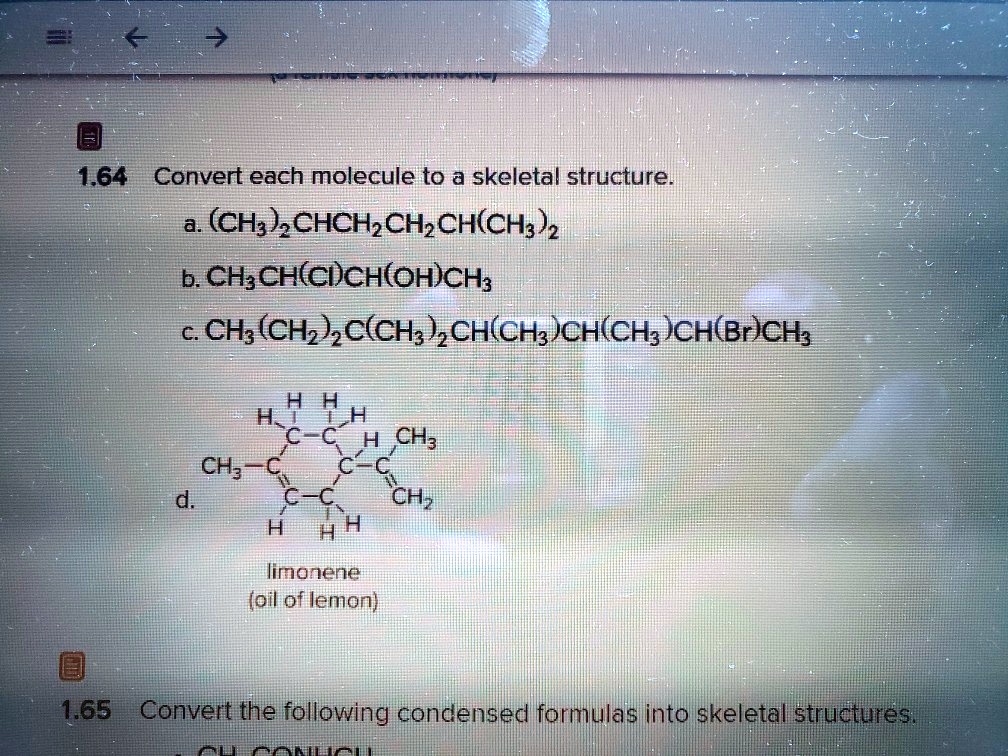
GIVE IUPAC NAMING OF FOLLLOWING COMPUNDS 1) CH3 CH(OH) CH2 CH3 2) CH3 CH2 CH(OH) CH2 CHO 3)CH3
This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Question: Based only on intermolecular forces, which of the following would be the least soluble in CH3CH (OH)CH3 ? A) CH3OCH3 B) CH3CH2CH3 C) H2O D) CH3CH2CH2OH. Here's the best way to solve it.
The total number of stereoisomers of the compound CH3CH=CH CH(OH) CH=CH CH3
Solution Verified by Toppr IUPAC name of CH 3 −CH (C2H 5)−CH 2 −CH (OH)−CH 3 is 4-methyl -2- hexanol. The parent compound contains a 6 C atom chain and a hydroxyl group. Hence, it is a derivative of hexanol. The hydroxyl group is present on the second C atom and methyl substituent is present on the fourth C atom.

Ch3ch(oh)ch3 Structural Formula
Answers. A. CH 3 CH 3 B. CH 3 CH 2 CH 2 CH 2 CH 3. 2. 3. (CH 3) 2 CHCH 2 CH (CH 3 )CH 2 CH 3. 4. 5. Condensed chemical formulas show the hydrogen atoms (or other atoms or groups) right next to the carbon atoms to which they are attached. Line-angle formulas imply a carbon atom at the corners and..
Ch3ch(oh)ch3 Structural Formula
Reaction Information Word Equation Isobutylene + Water = Butan-1-Ol CH3CH2CCH3 + H2O = CH3CH2CH (OH)CH3 is a Synthesis reaction where one mole of Isobutylene [CH 3 CH 2 CCH 3] and one mole of Water [H 2 O] combine to form one mole of Butan-1-Ol [CH 3 CH 2 CH (OH)CH 3] Show Chemical Structure Image Reaction Type Synthesis Addition reaction Reactants
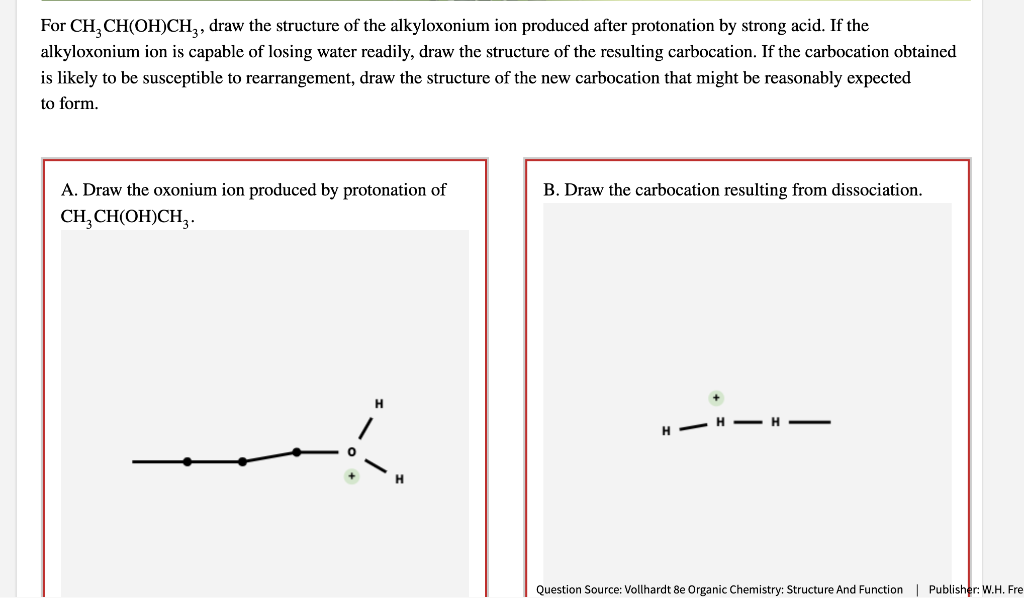
Solved For CH3CH(OH)CH3CH3CH(OH)CH3, draw the structure of
Chemistry Chemistry questions and answers Isopropyl alcohol, CH3CH (OH)CH3, is classified as a O primary alcohol. O secondary alcohol. O tertiary alcohol. O glycol. Question 2 The number of isomers of C4H2OH which are primary alcohols is: 2 Question 3 Which of the following compounds is a primary alcohol?

Solved Isopropyl alcohol, CH3CH(OH)CH3, is classified as a O
Made with Explain Everything
PPT Chapter 14 & 15 Alcohols, Ethers, Thiols and Chirality PowerPoint Presentation ID5571235
Balanced Chemical Equation 5 CH 3 CHO + 3 I 2 + 4 NaOH → 4 HCOONa + H 2 O + 6 CH 3 I ⬇ Scroll down to see reaction info and a step-by-step answer, or balance another equation. Reaction Information Word Equation Acetaldehyde + Diiodine + Sodium Hydroxide = Sodium Formate + Water + Methyl Iodide

Lewis Structure of Propan2ol CH3CH(OH)CH3 YouTube
CH3CH(OH)CH(CH3)CH2CH3 [3-methyl-2pentanol]+ HCl=? Organic Chemistry. 2 Answers Al E. May 12, 2018 This appears to be an SN1 reaction. Given a strong acid in water, an oxonium ion will form, leave, the molecule will stabilize by undergoing a carbocation rearrangement, and the chloride ion will attack the electrophilic carbon..

How to Draw the Lewis Dot Structure for CH3CH3 YouTube
Balanced Chemical Equation CH 3 COOH + CH 3 CH (OH)CH 3 → CH 3 COOCH (CH 3) 2 + H 2 O Equation is already balanced. ⬇ Scroll down to see reaction info and a step-by-step answer, or balance another equation. Reaction Information Word Equation Acetic Acid + Isopropyl Alcohol = Valeric Acid + Water

Solved Isopropyl alcohol, CH3CH(OH)CH3, is classified as a O
Balanced Chemical Equation 3 CH 3 CH (CH 3 )CH (OH)CH 3 + 5 HBr → 5 CH 3 CH 2 CH 2 Br + 3 H 2 O ⬇ Scroll down to see reaction info and a step-by-step answer, or balance another equation. Reaction Information Word Equation Isoamyl Alcohol + Hydrogen Bromide = Propyl Bromide + Water
Ch3ch(oh)ch3 Structural Formula
Isopropyl Alcohol | (CH3)2CHOH or CH3CHOHCH3 or C3H8O | CID 3776 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.

Give the IUPAC name of CH3 CH2 CH(OH) CH3
CH3CH (OH)CH3 + Na = CH3CHONaCH3 + H2 is a Single Displacement (Substitution) reaction where two moles of Isopropyl Alcohol [CH 3 CH (OH)CH 3] and two moles of Sodium [Na] react to form two moles of Sodium Propan-2-Olate [CH 3 CHONaCH 3] and one mole of Dihydrogen [H 2] Show Chemical Structure Image Reaction Type Single Displacement (Substitution)

A stepbystep explanation of how to draw the CH3OCH3 Lewis Dot Structure (Diethyl ether). YouTube
1.How many stereoisomers of 2,3-butanediol, CH3CH (OH) CH (OH) CH3, are there? a. 1. b. 2. c. 3. d. 4. 2. Which carbon atomic orbitals overlap to form the C = O bond in acetone, (CH3) 2C = O? a. sp2 + p. b. sp2 + sp2. c. sp3 + sp2. d. sp3 + 2sp. 3. Which of the following substituents has the highest priority according to the Cahn-Ingold-Prelog.
[Solved] Draw the expanded structural formula for the condensed... Course Hero
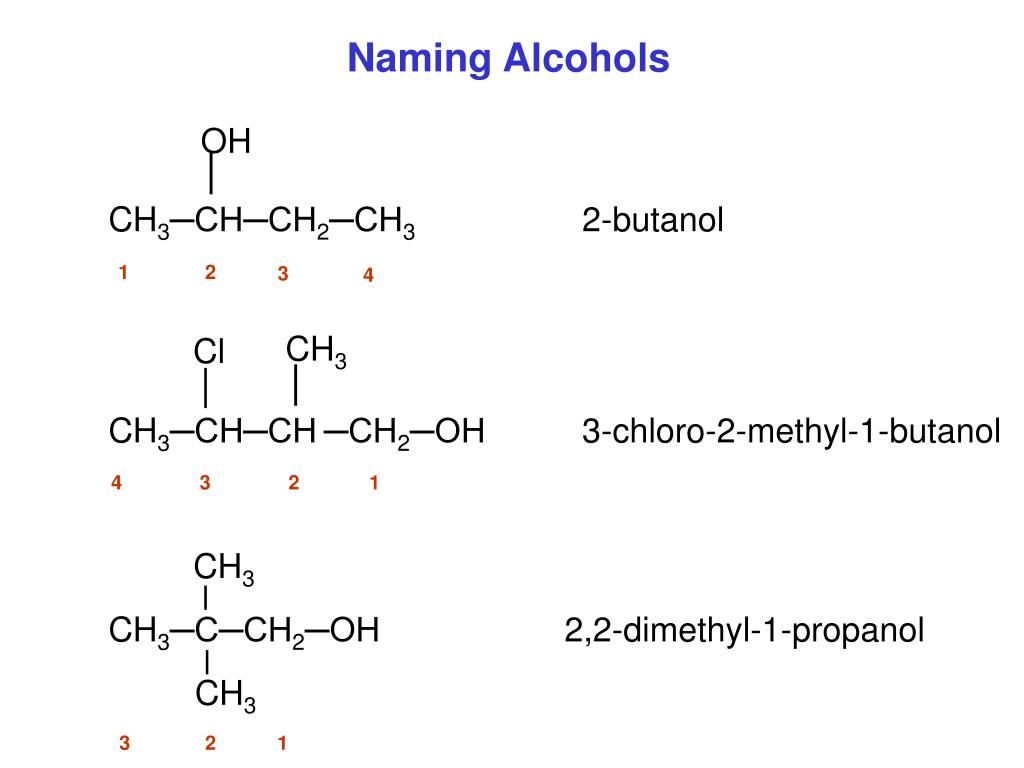
Solution. Ten carbon atoms in the LCC makes the compound a derivative of decane (rule 1), and the OH on the third carbon atom makes it a 3-decanol (rule 2).. The carbon atoms are numbered from the end closest to the OH group. That fixes the two methyl (CH 3) groups at the sixth and eighth positions.The name is 6,8-dimethyl-3-decanol (not 3,5-dimethyl-8-decanol).

Ch3ch(oh)ch3 Structural Formula
CH3CH (CH3) CH3. Methyl group in bracket (CH3) represents that it is attached to the second carbon atom. Acc. to IUPAC nomenclature the name of this compound is 2-methyl propane. its common name is isobutane as it is a chain isomer (structural isomer) of normal butane. VOTE.

Ch3ch Oh Ch3 Kmno4 H2so4 Margaret Wiegel
Example 3; Give the common and IUPAC names for each compound. CH 3 CH 2 CH 2 Br (CH 3) 2 CHCl; SOLUTION. The alkyl group (CH 3 CH 2 CH 2 -) is a propyl group, and the halogen is bromine (Br). The common name is therefore propyl bromide. For the IUPAC name, the prefix for bromine (bromo) is combined with the name for a three-carbon chain (propane), preceded by a number identifying the carbon.